Plan for a healthy future

Isotonix® Digestive Enzymes
sku 713037
$61.25 AUD
$1.11 AUD Cashback
This product qualifies for free or reduced cost delivery. Learn More
Benefits
- Promotes healthy digestion
- Supports the digestion of nutrients
- Relieve symptoms of stomach upsets*
- Enhance immune system function
- Contains DigeZyme®†, a multi-enzyme blend of amylase, protease, cellulase, tilactase, and lipase, and additional Amylase, formulated to replenish essential digestive enzymes
- One serving supplies over 100 mg of key digestive enzymes
- This vegan product contains no added wheat, soy, yeast, gluten, artificial flavour, salt, preservatives or milk
- Offered with the fastest and most efficient delivery system of all nutraceuticals – Isotonix
*If symptoms persist, seek the advice of a healthcare professional.
†DigeZyme® is a registered trademark of the Sabinsa Corporation.

Product Classifications
Gluten-Free - The finished product contains no detectable gluten (<10ppm gluten)
Vegan - This product is vegan
Isotonic-Capable Drinkable Supplements - Easy-to-swallow supplements in liquid form are immediately available to the body for absorption
Quality Standards - GMP Operations and Standardised Ingredients
Checked For: Heavy Metals, Microbiological Contaminants, Allergens, Potency, Purity and Identity
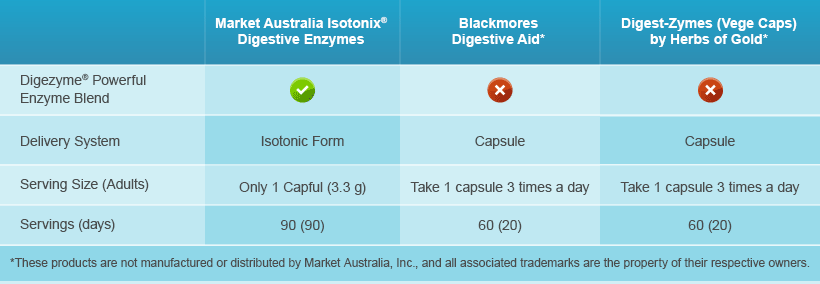
Compare
Keep your digestive system on track!
Isotonix® Digestive Enzymes is an isotonic-capable food supplement that is made from a unique blend of digestive enzymes. By including patented ingredients Digezyme® (a powerful blend of amylase, protease, cellulase, lactase and lipase) and additional amylase all designed to replenish essential digestive enzymes. Enzymes are important for the body’s proper absorption and utilisation of food. One serving supplies over 100 mg of key digestive enzymes. Over time, the body’s ability to make certain enzymes decreases as part of the natural aging process. Isotonix® Digestive Enzymes promotes healthy digestion and the digestion of nutrients which can support a healthy digestive tract. Our vegetarian formula also contains no added wheat, soy, yeast, gluten, artificial flavour, salt, preservatives or milk.
Why Choose Isotonix Digestive Enzymes?
Processed foods are the norm these days, not making it any easier for your body to digest and take in all the essential nutrients you need. Foods that would otherwise offer us their own added enzymes to help our bodies absorb more nutrients are increasingly processed, heated for extended shelf life and stripped of vital elements. Other poor eating habits and even aging can also contribute to inhibiting a healthy digestive process. This means our bodies may now need to work harder to absorb essential nutrients to keep us healthy and functioning. Isotonix Digestive Enzymes, an isotonic-capable food supplement, contains DigeZyme®, a multi-enzyme blend of amylase, protease, cellulase, tilactase, and lipase, and additional Amylase, which are formulated to replenish essential digestive enzymes, contributing to good digestive health. Isotonix Digestive Enzymes promotes healthy digestion and the digestion of nutrients, enhances immune system function, and relieves symptoms of stomach upsets. It utilises our advanced Isotonix® delivery system to provide you with essential nutrients that promote digestive health.
This digestive health promoting supplement features a formula of digestive enzymes, including DigeZyme®† – a blend of amylase, protease, cellulase, lactase and lipase. Digestive enzymes help your body break down food to utilise the complete spectrum of nutrients in the food you eat, resulting in a more complete digestive process and better nutritional absorption.
Isotonix Digestive Enzymes helps keep your digestive health on track! Its unique trademarked blend of digestive enzymes makes supporting your digestive health efficient and convenient.
Learn More
Isotonix Delivery System

Isotonic, which means “same pressure,” bears the same chemical resemblance of the body’s blood, plasma and tears. All fluids in the body have a certain concentration, referred to as osmotic pressure. The body’s common osmotic pressure, which is isotonic, allows a consistent maintenance of body tissues. In order for a substance to be absorbed and used in the body’s metabolism, it must be transported in an isotonic state.
Isotonix dietary supplements are delivered in an isotonic solution. This means that the body has less work to do in obtaining maximum absorption. The isotonic state of the suspension allows nutrients to pass directly into the small intestine and be rapidly absorbed into the bloodstream. With Isotonix products, little nutritive value is lost, making the absorption of nutrients highly efficient while delivering maximum results.
Ingredients
Amylase
Our formula uses Amylase within the DigeZyme® multi-enzyme complex as well as added amylase to assist more with digesting carbohydrates.
Amylases are enzymes that catalyze the hydrolysis of alpha-1, 4-glycosidic linkages of polysaccharides to yield dextrins, oligosaccharides, maltose and D-glucose. Amylases are derived from animal, fungal and plant sources. There are several different amylases. These enzymes are classified according to the manner in which the glysosidic bond is attacked. Alpha-amylases hydrolyze alpha-1, 4-glycosidic linkages, randomly yielding dextrins, oligosaccharides and monosaccharides. Alpha-amylases are endo-amylases. Exoamylases hydrolyze the alpha-1, 4-glycosidic linkage only from the non-reducing outer polysaccharide chain ends. Exoamylases include beta-amylases and glucoamylases (gamma-amylases, amyloglucosidases). Beta-amylases yield beta-limit dextrins and maltose. Gamma-amylases yield glucose. Amylases are used as digestants. Amylase activity is expressed as Dextrinizing Units or DU.
Protease
Proteases are enzymes that break peptide bonds between amino acids in proteins. The process is called proteolytic cleavage, a common mechanism of activation or inactivation of enzymes especially involved in blood coagulation or digestion. They use a molecule of water for this and are thus classified as hydrolases.
Proteases occur naturally in all organisms and constitute one to five percent of the gene content. These enzymes are involved in a multitude of physiological reactions from simple digestion of food proteins to highly regulated cascades (e.g. the blood clotting cascade, the complement system, apoptosis pathways and the invertebrate prophenoloxidase activating cascade). Peptidases can break either specific peptide bonds (limited proteolysis), depending on the amino acid sequence of a protein, or break down a complete peptide to amino acids (unlimited proteolysis). The activity can be a destructive change abolishing a protein's function or digesting it to its principal components, an activation of a function or a signal in a signalling pathway.
Lipase
A lipase is a water-soluble enzyme that catalyses the hydrolysis of ester bonds in water–insoluble, lipid substrates. Most lipases act at a specific position on the glycerol backbone of a lipid substrate (A1, A2 or A3). In the example of human pancreatic lipase (HPL), which is the main enzyme responsible for breaking down fats in the human digestive system, a lipase acts to convert triglyceride substrates found in oils from food to monoglycerides and free fatty acids.
Pancreatic lipases are found in the spaces outside of cells and have roles in the metabolism, absorption and transport of lipids throughout the body. Lipases are involved in diverse biological processes ranging from routine metabolism of dietary triglycerides to cell signalling and inflammation. Several different types of lipases are found in the human body, including pancreatic lipase, hepatic lipase, lysosomal lipase, gastric lipase, endothelial lipase and as various phospholipases.
Cellulase
Cellulase is an enzyme that breaks down cellulose to beta-glucose. Most animals (including humans) do not produce cellulase in their bodies, and are therefore unable to use most of the energy contained in plant material.
Cellulase is an enzyme derived from the fungi Aspergillus niger and Trichoderma longbrachiatum or other sources. Cellulose is an indigestible plant polysaccharide. It is the principal constituent of the cell wall of plants. Cellulase has cellulolytic activity, meaning that it hydrolyses cellulose. Cellulase hydrolyses the beta-D-1, 4-glycosidic bonds of cellulose. Cellulase is used as a digestive aid, particularly in animals, and for the management of flatulence. The activity of cellulase is expressed in cellulose units or CU.
Tilactase (Lactase)
Tilactase is a sugar-splitting enzyme that hydrolyzes lactose, a milk sugar, to produce glucose and galactose. In humans, tilactase is present predominantly along the brush border membrane of the differentiated enterocytes lining the villi of the small intestine. Tilactase is essential for the digestion of lactose in milk. Deficiency of the enzyme causes lactose intolerance. Symptoms of lactose intolerance include abdominal bloating and cramps, flatulence, diarrhea and nausea.
FAQ
What are digestive enzymes?
Digestive enzymes are special catalytic proteins that help your body break down food to utilise the complete spectrum of nutrients in the food we eat. Isotonix Digestive Enzymes acts to supplement and maximise the activity of the body’s own enzymes in an easy-to-take, pleasant-tasting drink.
Our lifestyles and diets are constantly changing. If the last 25 years are any indication, these changes are not usually for the best. Foods that would otherwise offer us their own added enzymes to help our bodies absorb more nutrients are increasingly processed, heated for extended shelf life and stripped of vital elements. The problem is that in making increasing numbers of foods “safe” for ingestion, we are in some cases making foods less healthy for our systems. Isotonix Digestive Enzymes provides the body with additional, essential enzymes necessary for maximum absorption of nutrients from the food we eat.
How do enzymes work in the body?
Enzymes are the workhorses of our cells. They are proteins that catalyse many thousands of biochemical reactions in the body. While most enzymes work inside our cells, digestive enzymes operate outside the cells in the gastrointestinal tract.
The start of digestion begins with digestive enzymes secreted by salivary gland cells into our mouths. Cells lining the gastrointestinal tract also contribute enzymes such as pepsin in the stomach. In addition, digestive enzymes are produced in the pancreas and are emptied into the upper part of the small intestine.
These enzymes help to break apart proteins, allowing the body to optimise its effort to digest proteins from plant and animal sources as well as break down starch, lactose, fats, and nucleic acids (DNA and RNA). The result is a more complete digestive process, resulting in better nutritional absorption.
Isotonix Digestive Enzymes supplies enzymes that are not inactivated by stomach acid. What this means is that the supplemental enzymes mix with and work in concert with the ingested food and begin to work with the body’s own digestive enzymes to release as many of the nutrients as possible.
What happens when we eat?
Even before we eat our body’s digestive action begins to take place. Simply smelling food activates our salivary glands (“mouth watering”). As the food enters the stomach, the stomach acid and pepsin work together to begin breaking the food down into material the small intestine (where most nutrients are absorbed) can use. Enzymes specific to each of the three nutrient groups are released at this stage, further breaking down the food and contributing to the digestive and absorption processes. These processes continue into the large intestine until the food’s nutritional content is extracted by the body.
Who should use Isotonix Digestive Enzymes?
Isotonix Digestive Enzymes is a beneficial supplement for all adults (18 years and over) looking to support their digestive health, especially those whose lifestyle consists of processed and fast foods. This is because such foods require the body to work harder to break down processed foods.
What are the different types of digestive enzymes?
There are three basic food enzymes, each specifically targeting the digestion of a different kind of food molecule: Protease, which helps digest proteins, amylase, which helps digest starch, and lipase, which helps digest fats. There are also four specialty enzymes: lactase (helps digest the sugar lactose in dairy products), maltase (helps digest the sugar maltose in foods), sucrase (helps digest table sugar and sugar found in fruits), and cellulase (helps digest cellulose fibers).
Each of these enzymes plays a significant part in the body’s overall health by helping to release specific and necessary nutrients into our bodies.
Is this a vegetarian product?
Yes.
Science
- Afonso, C. L., E. R. Tulman, Z. Lu, E. Oma, G. F. Kutish, and D. L. Rock. 1999. The genome of Melanoplus sanguinipes entomopoxvirus. J Virol 73:533-52.
- Anthony H, Collins CE, Davidson G, et al. Pancreatic enzyme replacement therapy in cystic fibrosis: Australian guidelines. J Pediatr—Child Health. 1999; 35:125-129.
- Barrett A.J., Rawlings ND, Woessner JF. The Handbook of Proteolytic Enzymes, 2nd ed. Academic Press, 2003. ISBN 0120796104.
- Billigmann P. [Enzyme therapy—an alternative in treatment of herpes zoster. A controlled study of 192 patients]. [Article in German]. Fortschr Med. 1995; 113:43-48.
- Bock U, Kolac C, Borchard G, et al. Transport of proteolytic enzymes across Caco-2 cell monolayers. Pharm Res. 1998; 15:1393-1400.
- Brady, L., A. M. Brzozowski, Z. S. Derewenda, E. Dodson, G. Dodson, S. Tolley, J. P. Turkenburg, L. Christiansen, B. Huge-Jensen, L. Norskov, and et al. 1990. A serine protease triad forms the catalytic centre of a triacylglycerol lipase. Nature 343:767-70.
- Carriere, F., C. Withers-Martinez, H. van Tilbeurgh, A. Roussel, C. Cambillau, and R. Verger. 1998. Structural basis for the substrate selectivity of pancreatic lipases and some related proteins. Biochim Biophys Acta 1376:417-32.
- Chapin III, F.S., P.A. Matson, H.A. Mooney. Principles of Terrestrial Ecosystem Ecology. Springer-Verlag New York, NY. 2002.
- Coenen TMM, Bertens AMC, De Hoog SCM, Verspeek-Rip CM. Safety evaluation of a lactase enzyme preparation derived from Kluyveromyces lactis. Food Chem Toxicol. 2000; 38:671-677.
- de Smet PA, Pegt GW, Meyboom RH. [Acute circulatory shock following administration of the non-regular enzyme preparation Wobe-Mugos]. [Article in Dutch]. Ned Tijdschr Geneeskd. 1991; 135:2341-2344.
- Diaz, B. L., and J. P. Arm. 2003. Phospholipase A(2). Prostaglandins Leukot Essent Fatty Acids 69:87-97.
- Dominguez-Munoz JE, Birckelbach U, Glassbrenner B, et al. Effect of oral pancreatic enzyme administration on digestive function in healthy subjects: comparison between two enzyme preparations. Aliment Pharmacol Ther. 1997; 11:403-408.
- Eckert K, Grabowska E, Stange R, et al. Effects of oral bromelain administration on the impaired immunocytotoxicity of mononuclear cells from mammary tumor patients. Oncol Rep. 1999; 6:1191-1199.
- Egmond, M. R., and C. J. van Bemmel. 1997. Impact of Structural Information on Understanding of Lipolytic Function, p. 119-129, Methods Enzymol vol. 284.
- Farkas G, Takacs T, Baradnay G, Szasz Z. [Effect of pancreatin replacement on pancreatic function in the postoperative period after pancreatic surgery]. [Article in Hungarian]. Orv Hetil. 1999; 140:2751-2754.
- Gilbert B, Rouis M, Griglio S, de Lumley L, Laplaud P. 2001. Lipoprotein lipase (LPL) deficiency: a new patient homozygote for the preponderant mutation Gly188Glu in the human LPL gene and review of reported mutations: 75 % are clustered in exons 5 and 6. Ann Genet 44(1):25-32.
- Girod, A., C. E. Wobus, Z. Zadori, M. Ried, K. Leike, P. Tijssen, J. A. Kleinschmidt, and M. Hallek. 2002. The VP1 capsid protein of adeno-associated virus type 2 is carrying a phospholipase A2 domain required for virus infectivity. J Gen Virol 83:973-8.
- Goni FM, Alonso A. 2002 Sphingomyelinases: enzymology and membrane activity. FEBS Lett. 531(1):38-46.
- Greenberger NJ. Enzymatic therapy in patients with chronic pancreatitis. Gastrenterol Clin North Am. 1999; 28:687-693.
- Hedstrom L. Serine Protease Mechanism and Specificity. Chem Rev 2002;102:4501-4523.
- Heikinheimo, P., A. Goldman, C. Jeffries, and D. L. Ollis. 1999. Of barn owls and bankers: a lush variety of alpha/beta hydrolases. Structure Fold Des 7:R141-6.
- Hooper NM. Proteases in Biology and Medicine. London: Portland Press, 2002. ISBN 1855781476.
- Identification of a variant associated with adult-type hypolactasia. Nat Genet 2002;30: 233-7. Free text. PMID 11788828.
- Kaul R, Mishra BK, Sutrador P, et al. The role of Wobe-Mugos in reducing acute sequelae of radiation in head and neck cancers—a clinical phase-III randomized trial. Indian J Cancer. 1999; 36:141-148.
- Kiessling WR. [Anaphylactic reaction in enzyme therapy of multiple sclerosis]. [Article in German]. Fortschr Neurol Psychiatr. 1987; 55:385-386.
- Klein G, Kullich W. [Reducing pain by oral enzyme therapy in rheumatic diseases]. [Article in German]. Wien Med Wochenschr. 1999; 149:577-580.
- Lowe, M. E. 1992. The catalytic site residues and interfacial binding of human pancreatic lipase. J Biol Chem 267:17069-73.
- Olds LC, Sibley E. Lactase persistence DNA variant enhances lactase promoter activity in vitro: functional role as a cis regulatory element. Hum Mol Genet 2003 Sep 15; 12(18): 2333-40. Free text. PMID 12915462.
- Puente XS, Lopez-Otin C. A Genomic Analysis of Rat Proteases and Protease Inhibitors. Genome Biol 2004;14:609-622.
- Puente XS, Sanchez LM, Overall CM, Lopez-Otin C. Human and Mouse Proteases: a Comparative Genomic Approach. Nat Rev Genet 2003;4:544-558.
- Ross J, Jiang H, Kanost MR, Wang Y. Serine proteases and their homologs in the Drosophila melanogaster genome: an initial analysis of sequence conservation and phylogenetic relationships. Gene 2003;304:117-31.
- Rowan AD, Buttle DJ, Barrett AJ. The cysteine proteinases of the pineapple plant. Biochem J. 1990; 266:869-875.
- Schrag, J. D., and M. Cygler. 1997. Lipases and alpha/beta hydrolase fold. Methods Enzymol 284:85-107.
- Seyis I, Aksoz N. Production of lactase by Trichoderma sp.. Food Technol Biotechnol 2004;42:121–124. Free text.
- Solomon, Eldra P.; Berg, Linda R.; & Martin, Diana W. (2002). Biology (6th ed). Tho
- Spiegel, S., D. Foster, and R. Kolesnick. 1996. Signal transduction through lipid second messengers. Curr Opin Cell Biol 8:159-67.
- Stauder G, Ransberger K, Streichhan P, et al. The use of hydrolytic enzymes as adjuvant therapy in AIDS/ARC/LAS patients. Biomed Pharmacother. 1988; 42:31-34.
- Steffen C, Menzel J. [Enzyme breakdown of immune complexes]. [Article in German]. Z Rheumatol. 1983; 42:249-255.
- Steffen C, Smolen J, Miehlke K, et al. [Enzyme therapy in comparison with immune complex determinations in chronic polyarthritis]. [Article in German]. Z Rheumatol. 1985; 44:51-56.
- Svendsen, A. 2000. Lipase protein engineering. Biochim Biophys Acta 1543:223-238.
- The Merck Manual of Diagnosis and Therapy, Chapter 24
- Tjoelker, L. W., C. Eberhardt, J. Unger, H. L. Trong, G. A. Zimmerman, T. M. McIntyre, D. M. Stafforini, S. M. Prescott, and P. W. Gray. 1995. Plasma platelet-activating factor acetylhydrolase is a secreted phospholipase A2 with a catalytic triad. J Biol Chem 270:25481-7.
- Wald M, Olejár T, Pouková P, Zadinova M. Proteinases reduce metastatic dissemination and increase survival time in C57B16 mice with the Lewis lung carcinoma. Life Sciences. 1998; 63:PL237-243.
- Wald M, Závadová E, Pouková P, et al. Polyenzyme preparation Wobe-Mugos inhibits growth of solid tumors and development of experimental metastases in mice. Life Sciences. 1998; 62:PL43-48.
- Winkler, F. K., A. D'Arcy, and W. Hunziker. 1990. Structure of human pancreatic lipase. Nature 343:771-4.
- Withers-Martinez, C., F. Carriere, R. Verger, D. Bourgeois, and C. Cambillau. 1996. A pancreatic lipase with a phospholipase A1 activity: crystal structure of a chimeric pancreatic lipase-related protein 2 from guinea pig. Structure 4:1363-74.
- Wolf M, Ransberger K. [Effect of proteolytic enzymes on the reciprocal growth modification of normal and tumor tissues]. [Article in German]. Arch Geschwultstforsch. 1968; 31:317-331.
Reviews
Displaying 1 - 5 of 29
9/7/2023
by KarenF
Reduce discomfort and bloating
I tried the Isotonix digestive enzymes and found it made the dinning experience more pleasant. Taking it before and after meals helped reduce the bloating sensation, it was a pleasant tasting drink.
Response from Customer Service
10/7/2023
Dear Valued Customer,
Thank you for taking the time to share your results with our Isotonix Digestive Enzymes. We are happy to hear you enjoy this product!
Thank you again!
Market Australia
2/3/2023
by LilyC
Wonderful product to help with Digestion
Love this product! I have it after every lunch and dinner.
Response from Customer Service
20/3/2023
This review made our day! One of the benefits of Isotonix Digestive Enzymes is that it Promotes healthy digestion. We appreciate you taking the time to share your personal experience with us
Thank you, Market Australia
27/2/2022
by Anonymous
Mrs
Pretty good
Response from Customer Service
3/3/2022
Dear Valued Customer,
Thank you for taking the time to share your thoughts on our Isotonix Digestive Enzymes, we agree the product is pretty good! We are happy to hear you are pleased with the product!
Thank you again!
Market Australia
29/11/2021
by Anonymous
great
this product does work for me. will purchase again
19/9/2020
by GERMAINEK
Product Review
Such a great product! Helps my digestive system and hence my immune!!!
- Prev
- Next



